AP Chemistry
How do I write an electron configuration?
2 min read•Last Updated on July 11, 2024
Writing an Electron Configuration
An electron-configuration involves simply the number of electrons in the atom. The atom fills orbitals with electrons following this following diagram:
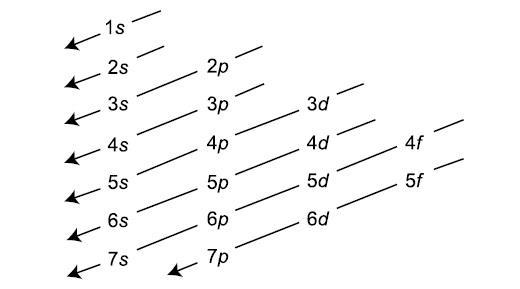
Each s level holds 2 electrons, each p level holds 6 electrons, each d level holds 10 electrons, and each f level holds 14 electrons.
Example
I will now show an example of how to write an electron-configuration for the atom lithium in its neutral state (unionized) A neutral lithium atom has 3 electrons.
We will start with the 1s orbital, giving it 2 electrons.
The electron-configuration then becomes 1s².
However, there is still 1 electron unaccounted for. This will reside in the next orbital: 2s.
The final electron-configuration, thus, for lithium becomes: 1s²2s¹.
Notice how the sum of the superscripts gives you the number of electrons.
Cations and Anions
Cations and anions can also be expressed through electron-configurations. To do so, simply add or subtract the number of electrons that were added or removed from the neutral atom.
For example, if atomic number 8 loses 1 electron to become a cation, the electron configuration will reduce by one, and will increase by one if it gains one electron.
Noble Gas Shortcuts
To further condense things, when the number of electrons gets tedious to write out, it is handy to refer to the nearest noble gas and go from there.
I will use the example of potassium.
Potassium has an electron-configuration of 1s²2s²2p⁶3s²3p⁶4s¹
The nearest noble gas is Argon, with electron-configuration 1s²2s²2p⁶3s²3p⁶.
Thus, the electron-configuration of potassium can be shortened to [Ar]4s¹
Practice
(See the end for answers!)
Want more practice? Make sure to read this study guide about AP Chemistry Practice FRQs!
(1) If you are given helium in a neutral state, what would the electron configuration be?
(2) What would the atom be if the electron-configuration was 1s²2s²2p⁶
(3) If you are given sodium as a cation with charge +1, what would the electron configuration be?
(4) What atom has the electron configuration [Kr] 5s¹?
Answers
(1) 1s²
(2) Atomic # 10: Neon
(3) Loses 1 electron, becomes 1s²2s²2p⁶, or just [Ne}
(4) Atomic #37: Rubidium
Want more resources? Make sure to check out: